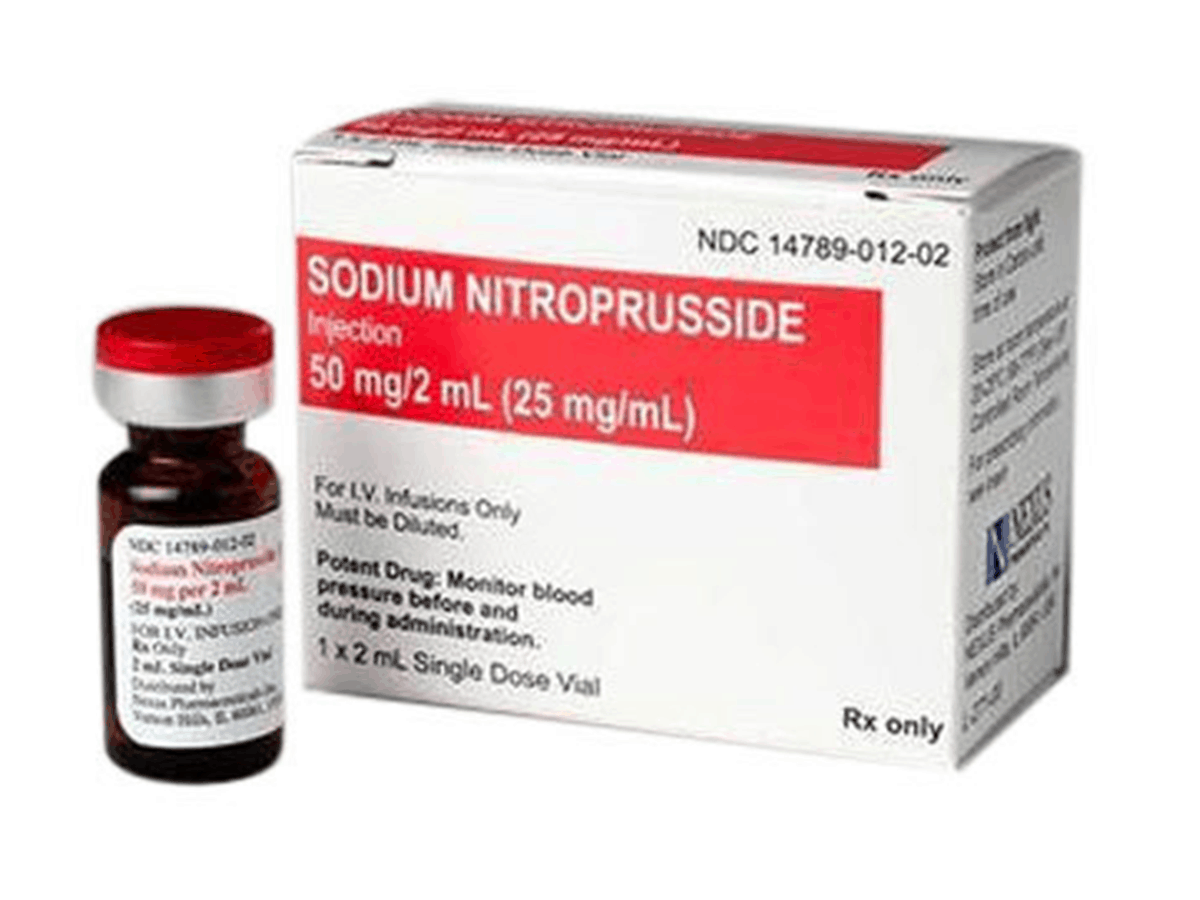
India/USA: Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DR REDDY, NYSE: RDY, along with its subsidiaries together referred to as “Dr. Reddy’s”) today announced the launch of Sodium Nitroprusside Injection, 50 mg/2 mL (25 mg/mL) Single-dose Vial, the therapeutic generic equivalent of Nitropress® (sodium nitroprusside) Injection, 50 mg/2mL vial, approved by the U.S. Food and Drug Administration (USFDA).
The Nitropress® brand and generics had U.S. sales of approximately $8 million MAT for the most recent twelve months ending in October 2019 according to IQVIA Health*.
Dr. Reddy’s Sodium Nitroprusside Injection is available in single-dose 50 mg/2 mL (25 mg/mL) vials.
Nitropress® is a trademark of Hospira, Inc.
| WARNINGS Sodium Nitroprusside Injection is not suitable for direct injection. The solution must be further diluted in sterile 5% dextrose injection before infusion. Sodium Nitroprusside can cause precipitous decreases in blood pressure (see DOSAGE AND ADMINISTRATION). In patients not properly monitored, these decreases can lead to irreversible ischemic injuries or death. Sodium nitroprusside should be used only when available equipment and personnel allow blood pressure to be continuously monitored. Except when used briefly or at low (< 2 mcg/kg/min) infusion rates, sodium nitroprusside gives rise to important quantities of cyanide ion, which can reach toxic, potentially lethal levels (see WARNINGS). The usual dose rate is 0.5 to10 mcg/kg/min, but infusion at the maximum dose rate should never last more than 10 minutes. If blood pressure has not been adequately controlled after 10 minutes of infusion at the maximum rate, administration of sodium nitroprusside should be terminated immediately. Although acid-base balance and venous oxygen concentration should be monitored and may indicate cyanide toxicity, these laboratory tests provide imperfect guidance. |
SIASAT NEWS