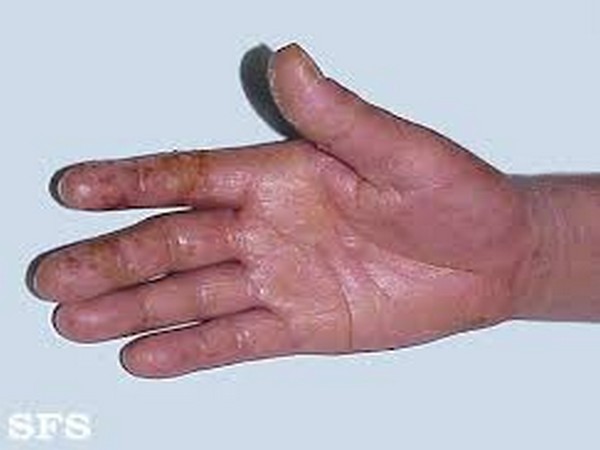
Washington: Patients with incurable hand disabling condition, called Dupuytren’s disease, have a ray of hope.
Dupuytren’s disease is a common condition that causes the fingers to curl irreversibly into the palm.
Researchers have found that an injection of the anti- Tumour Necrosis Factor (TNF) drug adalimumab into Dupuytren’s disease nodules results in reduction of the cell characteristics responsible for the progression of Dupuytren’s disease.
Based on the research, that TNF drives the development of myofibroblasts, the cell type that causes Dupuytren’s disease, the research team explored the effects of an anti-TNF drug injected directly into the Dupuytren’s nodule tissue. The results so far are very promising.
“Our data have shown that a concentrated formulation of adalimumab injected directly into the diseased tissue may be effective in targeting the cells responsible for Dupuytren’s disease,” said a researcher, Jagdeep Nanchahal.
“This brings new hope to people who suffer from this disabling condition, who currently have to wait for their situation to deteriorate, watching their hand lose function until it is bad enough for surgery. And then there’s the lengthy recovery ahead, a less than ideal situation to find yourself in,” he added.
This randomised trial (phase 2a) recruited 28 patients with Dupuytren’s disease who were scheduled to undergo surgery to remove diseased tissue in their hand. Two weeks prior to surgery they received a single injection of varying doses of the anti-TNF drug, or placebo.
The tissue removed during surgery, which is usually discarded, was then analysed in the laboratory. The team found that adalimumab (at a dose of 40mg formulated in 0.4ml) reduces expression of the fibrotic markers, -smooth muscle actin (-SMA) and type I procollagen, at 2 weeks post-injection, suggesting this drug could be used to stop the growth of disease-causing myofibroblast cells.
They also found the drug to be safe and well tolerated.
The anti-TNF drug adalimumab (Humira) is currently licensed in the EU for the treatment of a number of conditions including rheumatoid arthritis, Crohn’s disease and ulcerative colitis and overall has an excellent safety profile.
“We are delighted to have received further funding from the Wellcome Trust and Department of Health to test whether adalimumab will work for patients with early-stage Dupuytren’s disease,” said another researcher, Sir Marc Feldmann.
Marc and his team identified TNF as a therapeutic target in patients with rheumatoid arthritis.
“The Kennedy Institute has a long history of successfully translating laboratory findings to the clinic'” said another lead researcher, Fiona Powrie.
“This type of work requires close collaboration between laboratory scientists, clinical trialists and clinician scientists over many years,” she added.
The study appears in EBioMedicine journal.
(ANI)