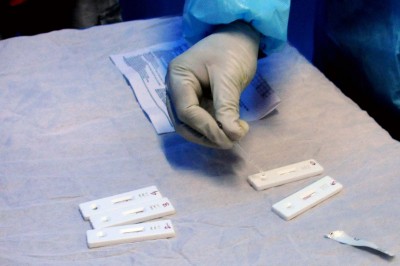
New York, Feb 24 : Researchers have developed two new rapid diagnostic tests for Covid-19 — one to detect Covid-19 variants and one to help differentiate from other illnesses that have Covid-like symptoms.
According to the findings published in the journal Bioengineering, the technology for both tests uses the cutting-edge CRISPR/Cas9 system.
Using commercial reagents, they describe a Cas9-based methodology for nucleic acid detection using lateral flow assays and fluorescence signal generation.
“The approval of the SARS-CoV-2 vaccine is highly promising, but the time between first doses and population immunity may be months,” said researcher Mark J. Osborn from the University of Minnesota.
“This testing platform can help bridge the gap between immunization and immunity,” Osborn added.
The first test is a rapid diagnostic test that can differentiate between Covid-19 variants and can be performed without specialized expertise or equipment, the researchers said.
It uses technology similar to at-home pregnancy testing and produces results in about an hour.
The second, more sensitive test allows researchers to analyze the same sample simultaneously for Covid-19 (SARS-CoV-2), Influenza A and B and respiratory syncytial virus by measuring fluorescence.
These viruses manifest with similar symptoms, so being able to detect and differentiate them adds a new diagnostic tool to slow the spread of Covid-19. This test also takes about an hour and could be easily scaled so many more tests can be performed.
The team is now seeking to enhance sensitivity and real-world application of this test in support of rapidly detecting and identifying Covid-19 variants.
In order to provide access to their new testing technology for healthcare providers and the public, the researchers are currently exploring ways to scale up and license their new diagnostics.
Disclaimer: This story is auto-generated from IANS service.