A Chennai-based health company called Global Pharma Healthcare has recalled its eye products from the US after its Food and Drug Administration (FDA) termed its eye drops as contaminated with a drug-resistant bacteria causing infections such as permanent loss of sight.
Around 55 US citizens were reported to have been infected by the bacteria, including one death.
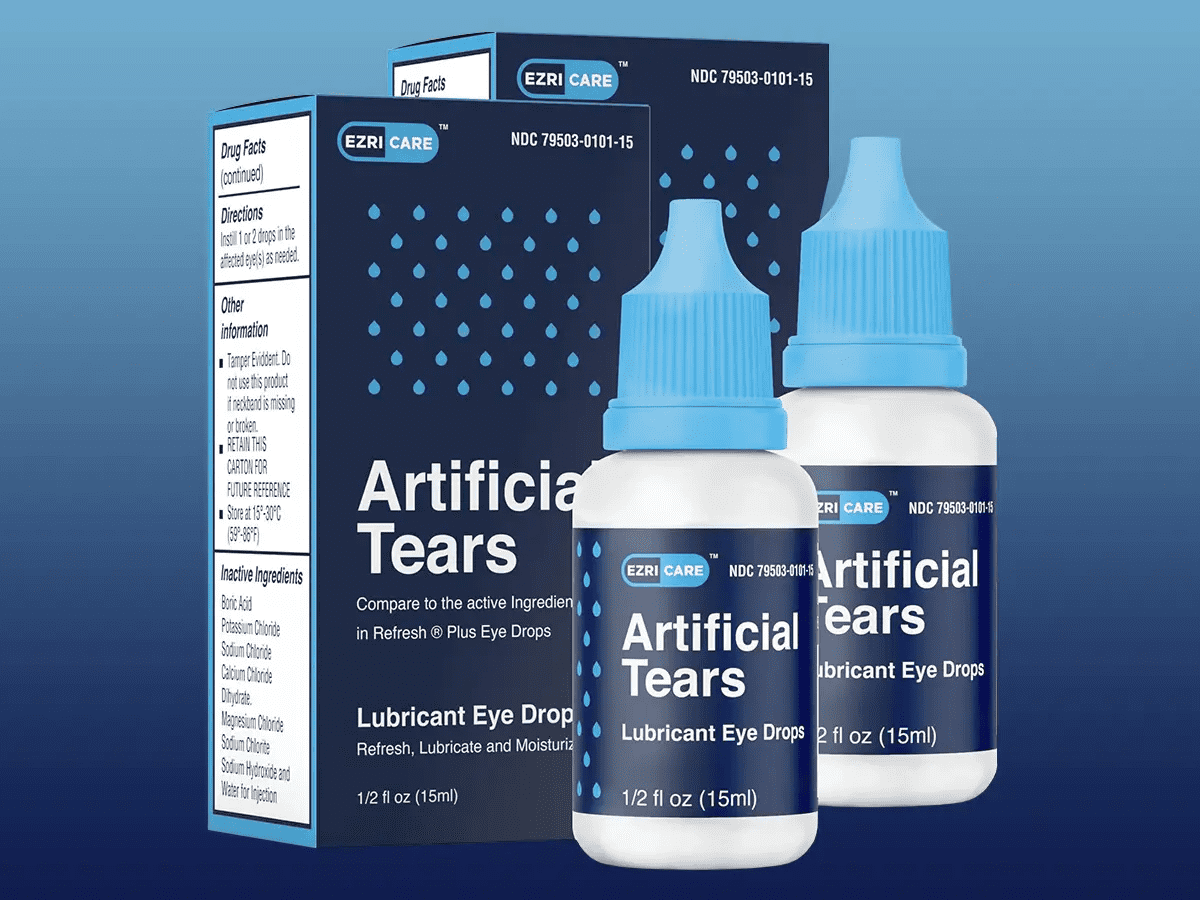
Global Pharma Healthcare Private Limited’s artificial tears were sold under the brand names EzriCare or Delsam Pharma. the company recalled its products on February 1.
Global Pharma Healthcare Private Limited has released a statement ensuring full cooperation in the investigations.
“Global Pharma is fully cooperating with US federal authorities and is continuing to investigate this matter, but thus far we have not determined whether our manufacturing facility is the source of the contamination,” a company spokesperson told ThePrint in an email.
“Nevertheless, out of an abundance of caution, we are recalling the products at issue. Our internal investigation is on,” the spokesperson added.
According to a CBS report, a spokesperson from the Centers for Disease Control and Prevention (CDC) confined that 11 patients who took the eye drug have lost vision in one eye.
The bacterium usually spreads to people in hospitals or other healthcare settings when they’re exposed to contaminated water or soil, where it typically lives, according to the CDC.

